
Problem 2: The quarterly payment on a 15‐year construction loanĭiscuss the types of Hetropolysaccharides.A woman uses a contact lens in order to see with a power or.Frederick has two productionīall is thrown vertically upwards with a speed of 20 m / s. Frederick Company has two service departments (Cafeteria.Imagine that you are a scientist interested in the population.

Imagine you are the owner of a natural gas company.What is the molarity of this MgCl2 solution? MgCl2 has a molar mass of 95.Īn aqueous solution of MgCl2 that is 27.5% (mass%) and has a An aqueous solution of MgCl2 that is 27.5% (mass%) and has a solution density of 1.25 g/mL.How many mL of a 0.620 M solution will contain 0.250 moles of calcium chloride? Calculate the moles and the mass of solute present in 200. mL solution Calculate molarity of an aqueous solution containing 5.20 g ammonium sulfate in a 250.0 mL solution. (Xb for methylamine is 4.2 * 10-4) What is the pH at the equivalence.Ĭalculate the molarity of an aqueous solution containing 1.80 mole potassium chloride in a.Ĭalculate the molarity of an aqueous solution containing 1.80 mole potassium chloride in a 800. sample of 0.299 M methylamine, CH3NH2, is titrated with 0.331 M hydroiodic acid, HI at 25☌. One liter of this solution could be converted into a buffer by the addition of 1.0.27 mol NH4Br 6.0.27 mol HBC 6.0.13 mol HCI 1.0.26 mol NH3 9.0.26 mol KCI QUESTION 19 A 29.9 ml. QUESTION 18 An aqueous solution contains 0.27 M ammonium chloride, NH4Cl. please answer both questions QUESTION 18 An aqueous solution contains 0.27 M ammonium chloride, NH4Cl.The density of the solution is (1.400x10^0) g/mL and the solute hasĪ molar mass of (1.77x10^2) g/mol. An aqueous solution has a mass percent of solute of 18.4%. Of the solution is (1.150x10^0) g/mL and the solute has a molar An aqueous solution has a Molarity of 1.632 M. Is the mass % of the solute (percent by weight, also written

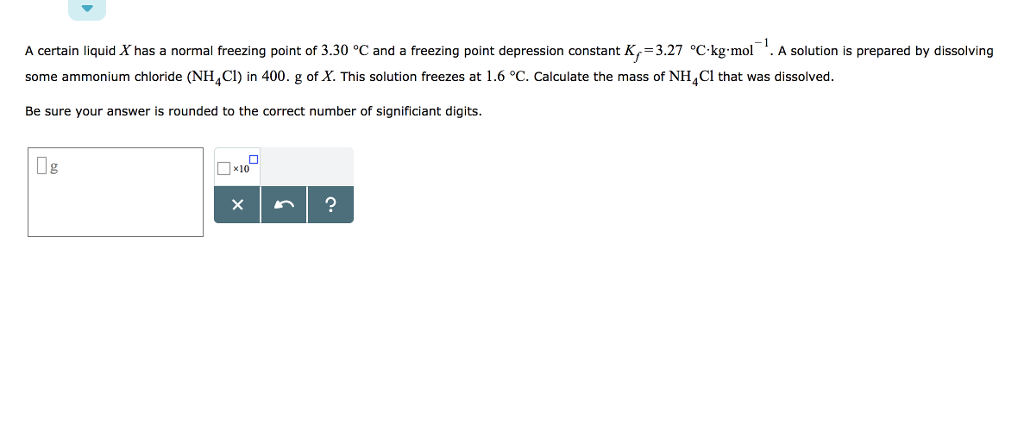
If the molarity of an aqueous solution of barium chloride isĥ.23 M, and the density of the solution is 1.20144 g/mL, then what If the molarity of an aqueous solution of barium chloride is.What is the molality of ammonium chloride in the solution? The formula weightof NH4Cl is 53.50 g/mol At 20☌, a 2.32 M aqueous solution of ammonium chloride has a density of 1.0344 g/mL.


 0 kommentar(er)
0 kommentar(er)
